Products
Integrity, passion, professionalism, respect and cooperation
Address: No. 1000, North of Shengzhou Avenue, Shengzhou City, Zhejiang Province, China
Tel: +86-575-83123228
Fax: +86-575-83101736
E-mail: hr@alkpharm.cn
URL: http://www.alkpharm.com
0.25g-6 tablets-2 boards Cefaclor Capsules

Cefaclor Capsules Instructions
Please read the instructions carefully and use them under the guidance of physician.
[Drug name]
Common name: Cefaclor Capsules
English name: Cefaclor Capsules
Chinese Pinyin: ToubaoKeluo Jiaonang
[Ingredients]
The active ingredient of this product is cefaclor. Chemical name: (6R ,7R)-7-[(R )-2-Amino-2-phenylacetamido]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0] oct- 2-ene-2-carboxylic acid monohydrate.
Structural formula:
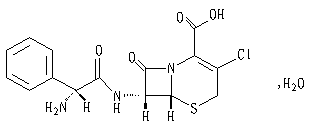
Molecular formula: C15H14ClN3O4S·H2O
Molecular weight: 385.82
[Properties]
The contents are white to yellowish powder.
[Indications]
This product is indicated in respiratory system, urinary system, otolaryngology, skin and soft tissue infection caused by sensitive bacteria.
[Specifications] 0.25g(as C15H14ClN3O4S)
[Usage and dosage]
This product is suitable for oral administration on an empty stomach
Adults usually use 0.25g (1 capsule) once a day, 3 times a day. The dose may be doubled in case of severe infection, but the total amount per day does not exceed 4g (16 capsules)), or as directed by your doctor.
Pediatric medication: Generally 20mg/kg per day, given in 3 doses, the dose can be increased to 40mg/kg in case of severe infection , but the total amount per day does not exceed 1g (4 capsules).
[Taboo]
It is contraindicated in those who are allergic to cefaclor and other cephalosporins.
[Use in pregnant and lactating women]
Pregnant women: It is not advisable for pregnant women to use this product unless it is necessary.
Childbirth: The effect of cefaclor on childbirth is unclear.
Lactating women: It is prudent to apply cefaclor to lactating women.
[Use in children]
The efficacy and safety of this product for infants within one month has not been established.
[Storage]
It is kept in closed container in shading (under 20℃) and dry place.
[Packing]
Aluminum plastic packaging:
6 capsules×1 board / box, 8 capsules×1 board / box, 10 capsules×1 board / box,
6 capsules×2 boards/ box, 8 capsules×2 boards/ box, 10 capsules×2 boards/ box.
[Validity period]
24 months
[Executive standards]
Chinese Pharmacopoeia 2015 Edition 2
[Approval Number]
State Medical Permitment No. H20093382
[Manufacturer]
Enterprise: Zhejiang Anglikang Pharmaceutical Co., Ltd.
Production address: No. 1000, North of Shengzhou Avenue, Shengzhou City, Shaoxing City, Zhejiang, China
P. C.: 312400
Tel: +86-575-83108588
Fax: +86-575-83101736

 Anglikang is a modern pharmaceutical company specializing in the health of Chinese people, featuring oral cephalosporins, cardiovascular and kidney diseases.
Anglikang is a modern pharmaceutical company specializing in the health of Chinese people, featuring oral cephalosporins, cardiovascular and kidney diseases. In the future development, Anglikang will adhere to the two-wheel drive of technological transformation and product innovation, and build the company into an innovative international enterprise with strong comprehensive competitiveness in the industry.
In the future development, Anglikang will adhere to the two-wheel drive of technological transformation and product innovation, and build the company into an innovative international enterprise with strong comprehensive competitiveness in the industry. Anglikang core values: integrity, passion, professionalism, respect, cooperation
Anglikang core values: integrity, passion, professionalism, respect, cooperation Anglikang always insists on technological innovation to drive enterprise development and continuously invests a large amount of research and development funds.
Anglikang always insists on technological innovation to drive enterprise development and continuously invests a large amount of research and development funds. 

