Products
Integrity, passion, professionalism, respect and cooperation
Address: No. 1000, North of Shengzhou Avenue, Shengzhou City, Zhejiang Province, China
Tel: +86-575-83123228
Fax: +86-575-83101736
E-mail: hr@alkpharm.cn
URL: http://www.alkpharm.com
2.5mg-7 tablets-2 boards Levamlodipine Besylate Tablets
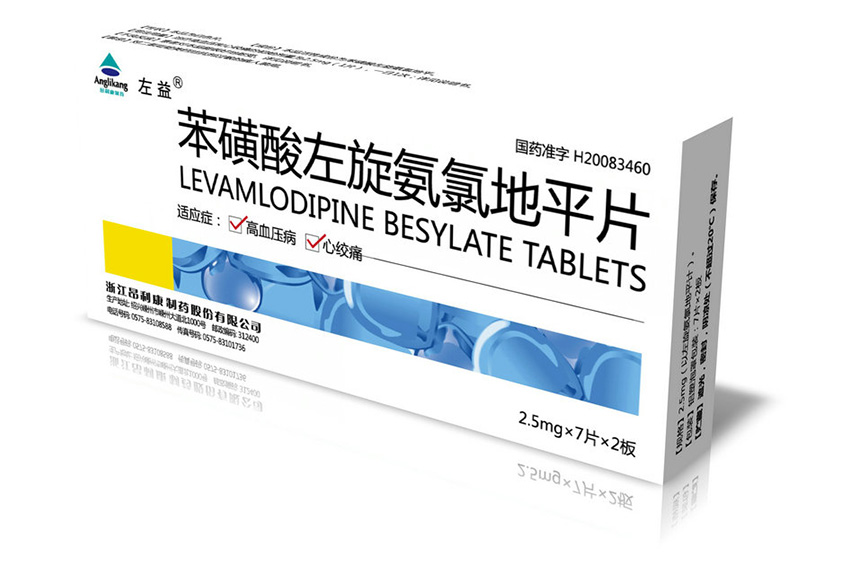
Levamlodipine Besylate Tablets Insturctions
Please read the instructions carefully and use them under the guidance of physician.
[Drug name]
Common name: Levamlodipine Besylate Tablets
English name: Levamlodipine Besylate Tablets
Chinese Pinyin: Benhuangsuan Zuoxuan’anlüdiping Pian
[Ingredients]
The active ingredient of this product is levamlodipine besylate.Chemical name: (S)-(-)-3-ethyl-5-methyl-2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dihydro-6- Base-3,5-pyridinedicarboxylate besylate.
Structural formula:
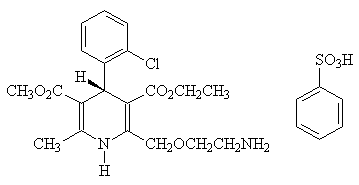
Molecular formula: C20H25 N2O5 Cl ×C6H6O3S
Molecular weight: 567.05
[Properties]
White tablets.
[Indications]
(1)High blood pressure(2)Angina.
[Specifications]
2.5mg(calculated as L-amlodipine)
[Usage and dosage]
(1)The initial dose for the treatment of hypertension and angina is 2.5 mg (1 tablet), once a day;Depending on the patient's clinical response, the dose can be increased to a maximum of 5 mg (2 tablets) once a day.
(2)Dose adjustment is not required when combined with thiazide diuretic, beta blocker and angiotensin converting enzyme inhibitor.
[Taboo]
It is contraindicated in patients with hypersensitivity to dihydropyridine calcium antagonists.
[Use in pregnant and lactating women]
This product is recommended only if there is no other safer alternative to the drug and the disease itself is more dangerous to the mother and child.
[Use in children]
No information is available on this product for children.
[Use in the elderly]
Normal doses in older patients.
[Storage]
Store in a well-closed container in shading place (under 20℃).
[Packing]
Aluminum-plastic blister packaging, 7 tablets×1 board / box, 7 tablets×2 boards/ box,7 tablets×4 boards/box, 10 tablets×1 board / box, 10 tablets×2 boards/ box.
[Validity period]
24 months
[Executive standards]
YBH05882008
[Approval Number]
State Medical Permitment No. H20083460
[Manufacturer]
Enterprise: Zhejiang Anglikang Pharmaceutical Co., Ltd.
Production address: No. 1000, North of Shengzhou Avenue, Shengzhou City, Shaoxing City, Zhejiang, China
P. C.: 312400
Tel: +86-575-83108588
Fax: +86-575-83101736

 Anglikang is a modern pharmaceutical company specializing in the health of Chinese people, featuring oral cephalosporins, cardiovascular and kidney diseases.
Anglikang is a modern pharmaceutical company specializing in the health of Chinese people, featuring oral cephalosporins, cardiovascular and kidney diseases. In the future development, Anglikang will adhere to the two-wheel drive of technological transformation and product innovation, and build the company into an innovative international enterprise with strong comprehensive competitiveness in the industry.
In the future development, Anglikang will adhere to the two-wheel drive of technological transformation and product innovation, and build the company into an innovative international enterprise with strong comprehensive competitiveness in the industry. Anglikang core values: integrity, passion, professionalism, respect, cooperation
Anglikang core values: integrity, passion, professionalism, respect, cooperation Anglikang always insists on technological innovation to drive enterprise development and continuously invests a large amount of research and development funds.
Anglikang always insists on technological innovation to drive enterprise development and continuously invests a large amount of research and development funds. 

